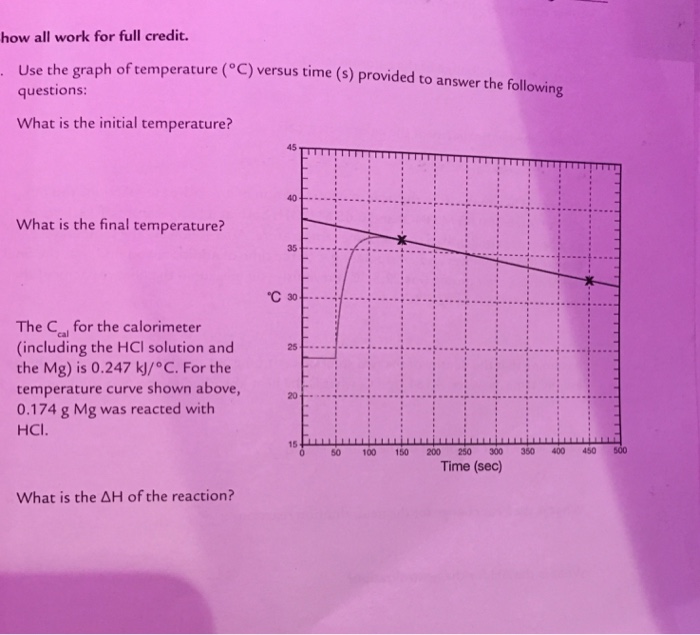
Temperature Vs Time Graph Calorimetry . Apply the first law of thermodynamics to calorimetry. Please combine all three runs on one graph. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. 1) the ice rises in temperature from −10.0 to 0.00 °c. Column a is time and. (for example, for mg, only 25.0 ml of hcl and. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. After dissolving the temperature of the solution is 10.26°c. 3) the liquid water then rises in temperature from zero to 100.0 °c. The solid ammonium nitrate and water have an initial temperature of 20.00°c. This is the initial temperature. 4) the liquid water then. Only one reactant is in the solution and so our reaction is not happening. A temperature versus time graph shows a decrease. 2) the ice melts at 0.00 °c.
from www.chegg.com
After dissolving the temperature of the solution is 10.26°c. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. Please combine all three runs on one graph. (for example, for mg, only 25.0 ml of hcl and. 3) the liquid water then rises in temperature from zero to 100.0 °c. This is the initial temperature. A temperature versus time graph shows a decrease. This video discusses the ins and out of temperature vs time graph of the physics of calorimetry. Apply the first law of thermodynamics to calorimetry. 1) the ice rises in temperature from −10.0 to 0.00 °c.
Solved Use the graph of temperature (degree C) versus time
Temperature Vs Time Graph Calorimetry 2) the ice melts at 0.00 °c. Temperature time for each of the three runs using excel. This is the initial temperature. Apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. 1) the ice rises in temperature from −10.0 to 0.00 °c. (for example, for mg, only 25.0 ml of hcl and. A temperature versus time graph shows a decrease. The solid ammonium nitrate and water have an initial temperature of 20.00°c. 2) the ice melts at 0.00 °c. After dissolving the temperature of the solution is 10.26°c. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. Only one reactant is in the solution and so our reaction is not happening. 3) the liquid water then rises in temperature from zero to 100.0 °c. Please combine all three runs on one graph.
From www.knowledgeboat.com
Chapter 11 Calorimetry Selina Solutions Concise Physics Class 10 Temperature Vs Time Graph Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. A temperature versus time graph shows a decrease. This video discusses the ins and out of temperature vs time graph of the physics of calorimetry. 3) the liquid water then rises in temperature from zero to 100.0 °c. Q = mcδt, where q is the. Temperature Vs Time Graph Calorimetry.
From www.youtube.com
HTPIB14D5 Specific Heats from Graph YouTube Temperature Vs Time Graph Calorimetry Temperature time for each of the three runs using excel. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. 1) the ice rises in temperature from −10.0 to 0.00 °c. 2) the ice melts at 0.00 °c. This is the initial temperature.. Temperature Vs Time Graph Calorimetry.
From chart-studio.plotly.com
Insulation Thermal Conductivity versus Mean Temperature scatter chart Temperature Vs Time Graph Calorimetry A temperature versus time graph shows a decrease. The solid ammonium nitrate and water have an initial temperature of 20.00°c. 2) the ice melts at 0.00 °c. Please combine all three runs on one graph. Column a is time and. This is the initial temperature. Only one reactant is in the solution and so our reaction is not happening. 4). Temperature Vs Time Graph Calorimetry.
From www.chegg.com
Solved Use the graph of temperature (degree C) versus time Temperature Vs Time Graph Calorimetry 4) the liquid water then. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Please combine all three runs on one graph. A temperature versus time graph shows a decrease. Temperature time for each of the three runs using excel. After dissolving the temperature of the solution is 10.26°c. This is the initial temperature.. Temperature Vs Time Graph Calorimetry.
From chart-studio.plotly.com
Temperature vs. Time scatter chart made by Tbuchta plotly Temperature Vs Time Graph Calorimetry 2) the ice melts at 0.00 °c. Only one reactant is in the solution and so our reaction is not happening. 1) the ice rises in temperature from −10.0 to 0.00 °c. Temperature time for each of the three runs using excel. (for example, for mg, only 25.0 ml of hcl and. 3) the liquid water then rises in temperature. Temperature Vs Time Graph Calorimetry.
From www.chegg.com
Chemistry Archive February 26, 2017 Temperature Vs Time Graph Calorimetry 2) the ice melts at 0.00 °c. The solid ammonium nitrate and water have an initial temperature of 20.00°c. 4) the liquid water then. After dissolving the temperature of the solution is 10.26°c. This is the initial temperature. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. 1). Temperature Vs Time Graph Calorimetry.
From www.youtube.com
MCAT Physics Temperature Vs Volume Graph Thermodynamics Temperature Vs Time Graph Calorimetry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. 3) the liquid water then rises in temperature from zero to 100.0 °c. 4) the liquid water then. 1) the ice rises in temperature from −10.0 to 0.00 °c. Apply the first law of thermodynamics to calorimetry. Q = mcδt, where q is the symbol. Temperature Vs Time Graph Calorimetry.
From www.youtube.com
BTec Calorimetry graph calculating a gradient YouTube Temperature Vs Time Graph Calorimetry (for example, for mg, only 25.0 ml of hcl and. Column a is time and. Please combine all three runs on one graph. The solid ammonium nitrate and water have an initial temperature of 20.00°c. 2) the ice melts at 0.00 °c. Only one reactant is in the solution and so our reaction is not happening. Apply the first law. Temperature Vs Time Graph Calorimetry.
From www.chegg.com
Solved QUESTION 2 4 pointsSave Answer Temperature vs. time Temperature Vs Time Graph Calorimetry A temperature versus time graph shows a decrease. Apply the first law of thermodynamics to calorimetry. 3) the liquid water then rises in temperature from zero to 100.0 °c. Please combine all three runs on one graph. 2) the ice melts at 0.00 °c. The solid ammonium nitrate and water have an initial temperature of 20.00°c. The thermal energy change. Temperature Vs Time Graph Calorimetry.
From www.chem.fsu.edu
Lab Report Temperature Vs Time Graph Calorimetry This video discusses the ins and out of temperature vs time graph of the physics of calorimetry. Column a is time and. The solid ammonium nitrate and water have an initial temperature of 20.00°c. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. This is the initial temperature. After dissolving the temperature of the. Temperature Vs Time Graph Calorimetry.
From www.youtube.com
Learn the Time/Temperature Graph for Water YouTube Temperature Vs Time Graph Calorimetry 1) the ice rises in temperature from −10.0 to 0.00 °c. Temperature time for each of the three runs using excel. This is the initial temperature. Column a is time and. 3) the liquid water then rises in temperature from zero to 100.0 °c. A temperature versus time graph shows a decrease. Compare heat flow from hot to cold objects. Temperature Vs Time Graph Calorimetry.
From www.numerade.com
SOLVED The following graph is a calibration curve made to determine Temperature Vs Time Graph Calorimetry Apply the first law of thermodynamics to calorimetry. 4) the liquid water then. 2) the ice melts at 0.00 °c. Please combine all three runs on one graph. 3) the liquid water then rises in temperature from zero to 100.0 °c. A temperature versus time graph shows a decrease. This video discusses the ins and out of temperature vs time. Temperature Vs Time Graph Calorimetry.
From chart-studio.plotly.com
Graph 2.Time Vs. Temperature scatter chart made by Cowas78 plotly Temperature Vs Time Graph Calorimetry A temperature versus time graph shows a decrease. Apply the first law of thermodynamics to calorimetry. 3) the liquid water then rises in temperature from zero to 100.0 °c. Please combine all three runs on one graph. This video discusses the ins and out of temperature vs time graph of the physics of calorimetry. (for example, for mg, only 25.0. Temperature Vs Time Graph Calorimetry.
From www.knowledgeboat.com
The diagram below shows the change of phases of a substance KnowledgeBoat Temperature Vs Time Graph Calorimetry 4) the liquid water then. Apply the first law of thermodynamics to calorimetry. The solid ammonium nitrate and water have an initial temperature of 20.00°c. The thermal energy change accompanying a chemical reaction is responsible for the change in temperature that takes place in a calorimeter. After dissolving the temperature of the solution is 10.26°c. This is the initial temperature.. Temperature Vs Time Graph Calorimetry.
From www.surfacesciencewestern.com
Differential Scanning Calorimetry (DSC) Surface Science Western Temperature Vs Time Graph Calorimetry 2) the ice melts at 0.00 °c. Apply the first law of thermodynamics to calorimetry. Temperature time for each of the three runs using excel. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Column a is time and. (for example, for mg, only 25.0 ml of hcl and. The solid ammonium nitrate and. Temperature Vs Time Graph Calorimetry.
From www.springboard.pro
Temperature vs time Springboard Temperature Vs Time Graph Calorimetry A temperature versus time graph shows a decrease. The solid ammonium nitrate and water have an initial temperature of 20.00°c. Apply the first law of thermodynamics to calorimetry. Please combine all three runs on one graph. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is. Temperature Vs Time Graph Calorimetry.
From www.youtube.com
Bomb Calorimetry Introduction Physical Chemistry Laboratory YouTube Temperature Vs Time Graph Calorimetry Column a is time and. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change in. 2) the ice melts at 0.00 °c. This is the initial temperature. Apply the first law of thermodynamics to calorimetry. 3) the liquid water then rises in temperature. Temperature Vs Time Graph Calorimetry.
From stock.adobe.com
DSC curve isolated on white. Differential scanning calorimetry Temperature Vs Time Graph Calorimetry 4) the liquid water then. This is the initial temperature. (for example, for mg, only 25.0 ml of hcl and. Only one reactant is in the solution and so our reaction is not happening. Q = mcδt, where q is the symbol for heat transfer (“quantity of heat”), m is the mass of the substance, and δt is the change. Temperature Vs Time Graph Calorimetry.